Stimulated Emission
LASER
LASER LASER is an abbreviation of Light Amplification by Stimulated Emission of Radiation. Lasers are light beams which are highly coherent and monochromatic & so powerful that they can travel miles into the sky, and they can also cut through the surfaces of metals. Theodore H Maiman at Hughes Research Laboratories was the first person to build a practical laser in 1960. Today lasers find applications in various fields and there are different types of lasers with numerous applications. Stimulated emission
Animation explaining stimulated emission and the laser principle In the classical view, the energy of an electron orbiting an atomic nucleus is larger for orbits further from the nucleus of an atom. However, quantum mechanical effects force electrons to take on discrete positions in orbitals. Thus, electrons are found in specific energy levels of an atom, two of which are shown below:
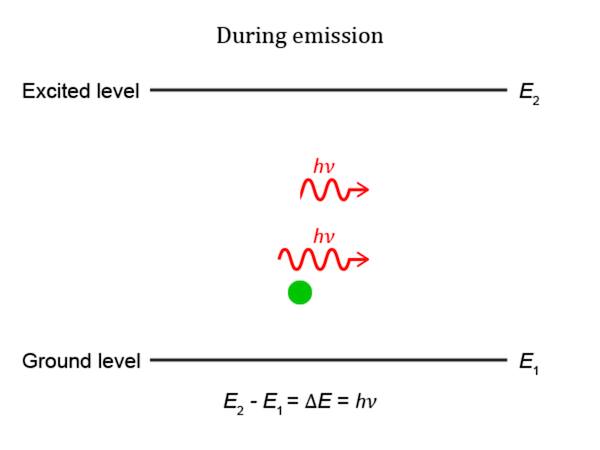
An electron in an atom can absorb energy from light (photons) or heat (phonons) only if there is a transition between energy levels that match the energy carried by the photon or phonon. For light, this means that any given transition will only absorb one particular wavelength of light. Photons with the correct wavelength can cause an electron to jump from the lower to the higher energy level. The photon is consumed in this process. When an electron is excited from one state to that at a higher energy level with energy difference ΔE, it will not stay that way forever. Eventually, a photon will be spontaneously created from the vacuum having energy ΔE. Conserving energy, the electron transitions to a lower energy level that is not occupied, with transitions to different levels having different time constants. This process is called spontaneous emission. Spontaneous emission is a quantum-mechanical effect and a direct physical manifestation of the Heisenberg uncertainty principle. The emitted photon has a random direction, but its wavelength matches the absorption wavelength of the transition. This is the mechanism of fluorescence and thermal emission. A photon with the correct wavelength to be absorbed by a transition can also cause an electron to drop from the higher to the lower level, emitting a new photon. The emitted photon exactly matches the original photon in wavelength, phase, and direction. This process is called stimulated emission.
Size of LASER (micrometre to big room size)