Thermodynamic System
Thermodynamics Part 1
Annotated color version of the original 1824 Carnot heat engine showing the hot body (boiler), working body (system, steam), and cold body (water), the letters labelled according to the stopping points in Carnot cycle.
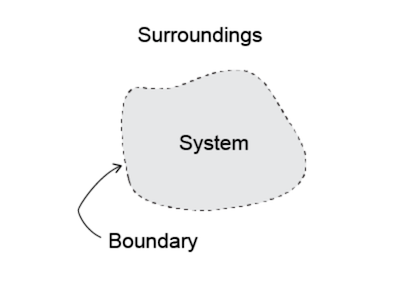
A diagram of a generic thermodynamic system An important concept in thermodynamics is the thermodynamic system, which is a precisely defined region of the universe under study. Everything in the universe except the system is called the surroundings. A system is separated from the remainder of the universe by a boundary which may be a physical or notional, but serve to confine the system to a finite volume. Segments of the boundary are often described as walls; they have respective defined ‘permeabilities’. Transfers of energy as work, or as heat, or of matter, between the system and the surroundings, take place through the walls, according to their respective permeabilities. Boundaries are of four types: fixed, movable, real, and imaginary. For example, in an engine, a fixed boundary means the piston is locked at its position, within which a constant volume process might occur. If the piston is allowed to move that boundary is movable while the cylinder and cylinder head boundaries are fixed. For closed systems, boundaries are real while for open systems boundaries are often imaginary. In the case of a jet engine, a fixed imaginary boundary might be assumed at the intake of the engine, fixed boundaries along the surface of the case and a second fixed imaginary boundary across the exhaust nozzle.