(click to open)
First Law
Thermodynamics Part 1
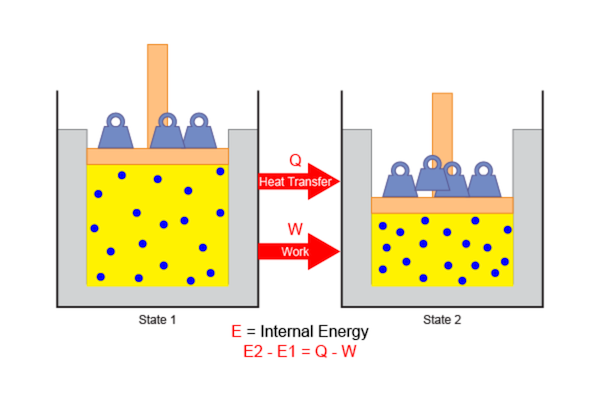
First Law of Thermodynamics First law of thermodynamics, also known as the law of conservation of energy, states that energy can neither be created nor destroyed, but it can be changed from one form to another. The first law of thermodynamics may seem abstract, but we will get a clearer idea if we look at a few examples of the first law of thermodynamics. First Law of Thermodynamics Examples: Plants convert the radiant energy of sunlight to chemical energy through photosynthesis. We eat plants and convert the chemical energy into kinetic energy while we swim, walk, breathe, and scroll through this page. Switching on light may seem to produce energy, but it is electrical energy that is converted.