(click to open)
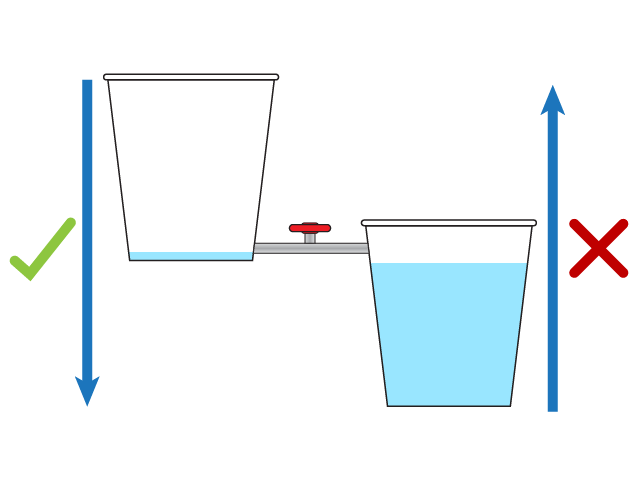
Water Flow
Thermodynamics Part 2
Second Law of Thermodynamics Second law of thermodynamics states that the entropy in an isolated system always increases. Any isolated system spontaneously evolves towards thermal equilibrium—the state of maximum entropy of the system. The entropy of the universe only increases and never decreases. Many individuals take this statement lightly and for granted, but it has an extensive impact and consequence. Visualizing the second law of thermodynamics If a room is not tidied or cleaned, it invariably becomes more messy and disorderly with time. When the room is cleaned, its entropy decreases, but the effort to clean it has resulted in increased entropy outside the room exceeding the entropy lost.