Third Law
Thermodynamics Part 2
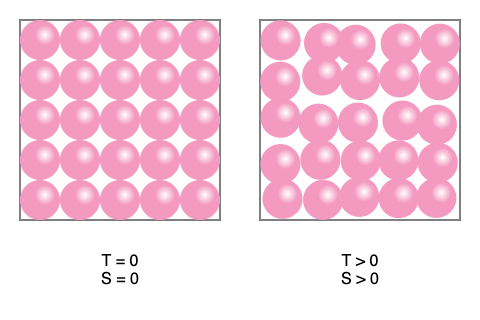
Third Law of Thermodynamics Third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero. The entropy of a pure crystalline substance (perfect order) at absolute zero temperature is zero. This statement holds true if the perfect crystal has only one state with minimum energy. Third Law of Thermodynamics Examples: Let us consider steam as an example to understand the third law of thermodynamics step by step: The molecules within it move freely and have high entropy. If one decreases the temperature below 100 °C, the steam gets converted to water, where the movement of molecules is restricted, decreasing the entropy of water. When water is further cooled below 0 °C, it gets converted to solid ice. In this state, the movement of molecules is further restricted and the entropy of the system reduces more. As the temperature of the ice further reduces, the movement of the molecules in them is restricted further and the entropy of the substance goes on decreasing. When the ice is cooled to absolute zero, ideally, the entropy should be zero. But in reality, it is impossible to cool any substance to zero.